Researchers Use Air-Tagged Macrophages to Transform Cancer Imaging, Diagnosis, and Treatment
July 10, 2025
By Chloe Arrington and Ashley Ritchie
Researchers at Georgia Tech have developed a new approach to cancer imaging and therapy.
The study, led by Costas Arvanitis, associate professor in the George W. Woodruff School of Mechanical Engineering and the Wallace H. Coulter Department of Biomedical Engineering, explores a noninvasive method for tracking immune cells in real time using ultrasound. The study was recently published in the journal Nature Communications.
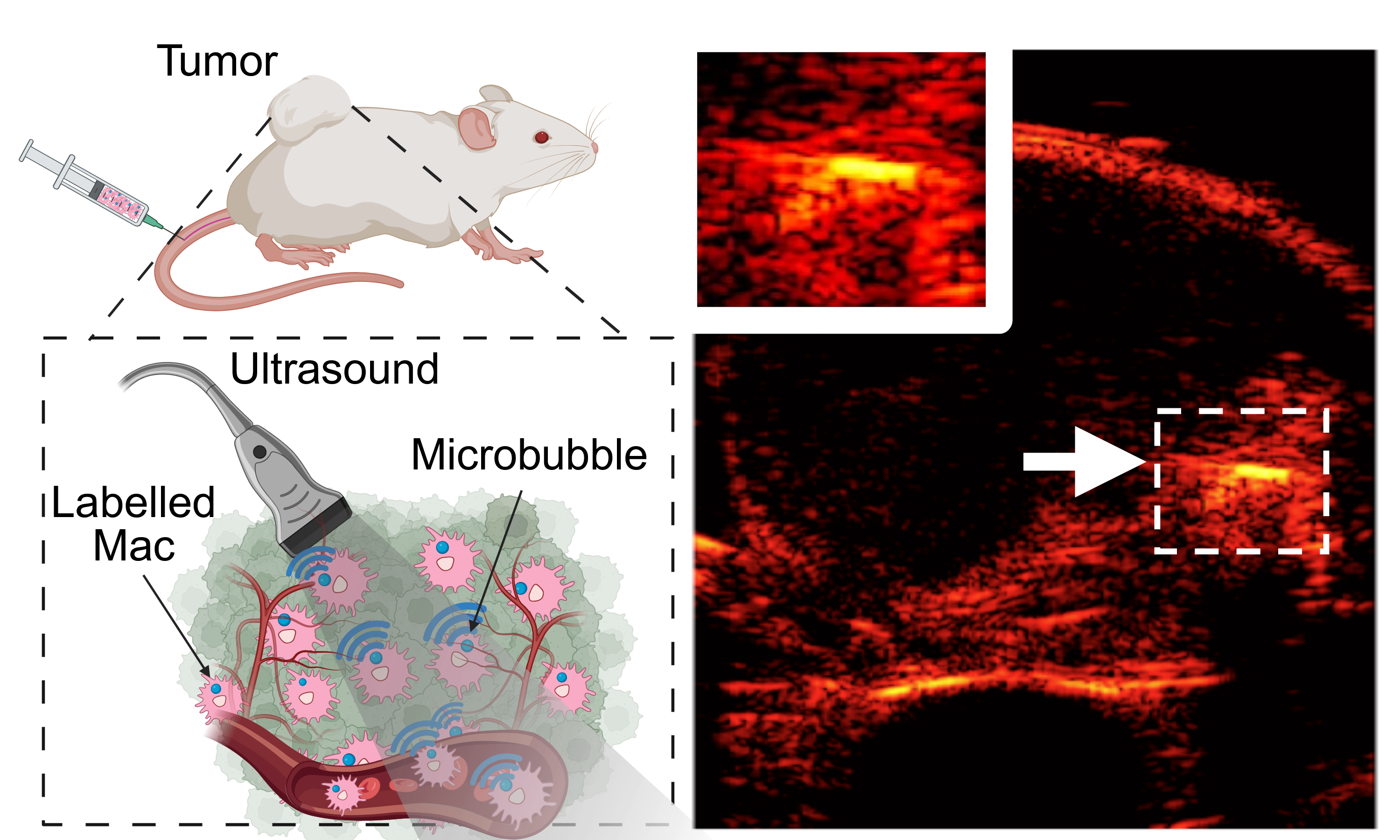
At the center of this innovation is the macrophage, a type of immune cell known for its diverse roles in the body's defense system. Arvanitis and his team have developed a technique to label these cells with FDA-approved, lipid-shelled microbubbles.
Once tagged, these macrophages can be visualized deep within tissues using linear and nonlinear ultrasound imaging. This allows observers to monitor how these cells move through the body and accumulate in tumors without compromising their viability or function.
This work builds on existing cancer immunotherapy and imaging research, addressing the limitations of other modalities like optical imaging, MRI, and PET. Unlike traditional imaging methods, this ultrasound-based approach combines high sensitivity (i.e., it approaches the theoretical limit of one cell), resolution, penetration depth, and safety. It is also compatible with wearable ultrasound devices that create new possibilities for imaging macrophage trafficking in solid tumors over long periods.
"The findings of this paper could have major implications for cancer diagnosis, prognosis, and therapy," said Arvanitis. By enabling high-resolution, real-time imaging of macrophage trafficking, the technology offers a powerful new tool for studying the tumor immune microenvironment, a key factor in cancer progression and treatment response.
Furthermore, the ability to package microbubbles inside immune cells may overcome existing challenges for ultrasound contrast agents, such as their short circulation time and inability to cross blood vessels to reach diseased tissues. It may also accelerate the discovery of new cancer biomarkers.
"The concept of injectable, engineered cell-based, active diagnostics is in striking contrast with current, passive, diagnostic modalities, which rely on uptake by the host’s cells of intravenously administered small molecule agents that lack the ability to overcome biological barriers and navigate through tissues, thereby providing a paradigm shift in disease diagnosis" said Arvanitis
This research also opens doors to immediate clinical applications for therapy. For instance, the method can directly guide image-based delivery of cell therapies into tumors, such as chimeric antigen receptor macrophages. It also has promising potential for precision drug delivery, where macrophages could carry therapeutic agents to tumors and release them on demand via ultrasound. Additionally, the technique may help assess tumor aggressiveness, detect micrometastases, and support novel microsurgical interventions.
The project is in collaboration with Associate Professor Brooks Lindsey and Professor Edward Botchwey in the Wallace H. Coulter Department of Biomedical Engineering. Lindsey provided materials and analysis tools, and Botchwey supported macrophage phenotype analysis.
As this research moves closer to clinical applications, it represents a promising step forward in the fight against cancer as well as other diseases, such as atherosclerosis.
Note: Chulyong Kim 1, Yann Ferry 2, Pranav Premdas 1, and Ashley Alva2 all contributed equally to this work.
1Woodruf School of Mechanical Engineering, 2Coulter Department of Biomedical Engineering
This study was supported by the NIH/NIBIB Grant R21EB037352 and NIH/NCI Grants R37CA239039 and R01CA273878.
